Learning Outline
Biological ChemistryPre-A&P
Basic Structural Concepts
Carbohydrates
Saccharides (C6H12O6)—sugar groups that make up many carbohydrates
- Monosaccharides—just one saccharide group
- Disaccharides—have two saccharide groups
- Examples: sucrose, maltose, lactose
- Also called “double sugars”
- Polysaccharides—have many saccharide groups
- Example: glycogen
Examples of function: fuel, fuel storage
Lipids
Triglycerides: three fatty acid “tails” and one glycerol holding them together
- Nonpolar; hydrophobic
- Fuel
Phospholipids: two fatty acid “tails” with a “head” containing glycerol and phosphate
- Tails are nonpolar; head is polar
- Form bilayers in water
- Form cellular membranes
- Source of steroid lipids
- For example, the body converts cholesterol into steroid hormones
- Stabilizes phospholipid bilayers
- Strengthens cellular membranes that are made up primarily of phospholipids
Proteins
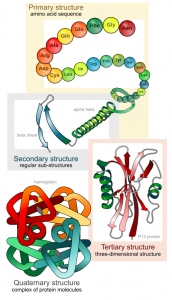
Protein structure
Proteins are folded/twisted chains of amino acids
- Proteins have 50 or more amino acids; polypeptides 10-50, peptides less than 10
- These different categories are used very loosely in real life
- Levels of protein structure (increasing degrees of structural complexity)
- Chaperone molecules assist proteins in folding properly
- The proper (functioning) shape of a protein is called its “native state”
- Denaturation is loss of proper 3D shape (native state)
- May be caused by extreme temp, extreme pH, chemical reactions, other extreme conditions
- Renatured proteins are those that have regained their native state after having been previously denatured
- Chaperone molecules may assist in refolding denatured proteins
- Misfolded proteins must be destroyed (and recycled) or they may cause damage
- May form deadly plaque (gunk)
- Categories of protein shape
- Fibrous proteins
- Globular proteins (globulus “small globe or sphere”)
- Nearly any shape that isn’t “fibrous”
- Also sometimes called globins
- Structure of molecule determines function (it’s all about shape)
- Protein folding has become a vital part of our understanding of how proteins function in the body
Types of Proteins
Structural proteins
- Form structures, e.g. collagen, keratin
Functional proteins
- Act as “chemists” or “regulators”, e.g. hormones, neurotransmitters, enzymes
- Enzyme action
- Lower “activation energy” of chemical reactions to body temp–that is, make chemistry happen that otherwise wouldn’t happen
- Lock-and-key model
- Enzyme fits substrate as a key fits into a lock
- Enzyme fits substrate as a key fits into a lock
- Active site
- Allosteric site (allosteric “shape changing” effect)
- Cofactors help enzymes
- Organic cofactors are coenzymes, e.g. vitamins
- Enzymes are specific (only work with one type of substrate)
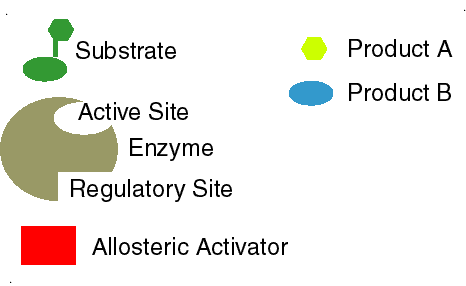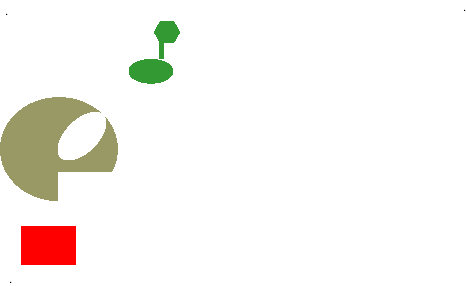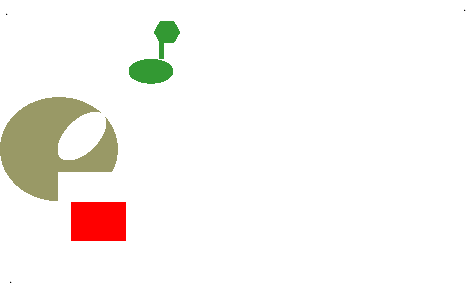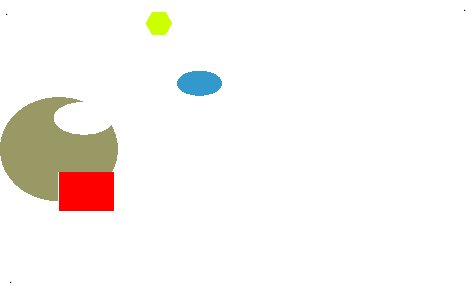
Simplified animation of the allosteric effect, in which shape affects function of enzymes
Nucleic acids
- Examples
- Encode genetic information
- Structure/function of DNA discovered in 1953 by
- James Watson
- Francis Crick
- Maurice Wilkins
- Rosalind Franklin
- Structure/function of DNA discovered in 1953 by
- Nucleotide is the subunit, made of three components
- Sugar (ribose in RNA; deoxyribose in DNA)
- Phosphate
- Nitrogen base
- RNA nucleotides link to form strands that sometimes fold
- Some RNA types do form double strands
- DNA nucleotides form a double strand that twists (double helix)
- Information is encoded in the sequence of nitrogen bases
- Every three bases is one codon or “word” in “genetish”
- Each codon represents one amino acid in a sequence to form a protein
- Genetish is what science author Matt Ridley calls the language of the genetic code
- A gene is a sequence of codons that tells a cell the correct order of amino acids in a polypeptide (protein)
- DNA is like a cookbook that contains recipes (genes) made up of words (codons) made up of different letters (nucleotide bases) that instruct the cell how to make a particular dish (specific protein)
- Every three bases is one codon or “word” in “genetish”
- ATP (adenosine triphosphate) is a modified nucleotide
- Breaking of last phosphate bond can release energy for cell work
- [Very] temporary energy-storage “battery” of cell
- Other modified nucleotides similarly transfer energy
- NAD, FAD, CP
- The role of each of these molecules will be discussed later in the course
Combined forms
- Molecule made up of more than one type of macromolecule
- Sometimes called “hybrid” molecules
- Examples:
- Glycoprotein
- Proteoglycan
- Glycolipid
- Ribonucleoprotein
- Lipoprotein
This is a Learning Outline page.
Did you notice the EXTRA menu bar at the top of each Learning Outline page with extra helps?
Last updated: August 14, 2022 at 17:01 pm