Learning Outline
Cell TransportPre-A&P
- Passive forms of transport — do not require cell expenditure of energy slide slide


- Particles move down their concentration gradient
- That is, particles move from area of high concentration to area of low concentration
- If possible, particles eventually reach a dynamic equilibrium in which there is no difference in concentration
Terms related to diffusion
Solution – liquid mixture, usually composed of a liquid solvent and one or more dissolved particles (solutes)
Solute – a dissolved particle
Solvent – a liquid into which other particles may dissolve
< EXAMPLE: seawater is a solution in which sea salts are the solutes dissolved in the solvent water >
Permeable – describes a structure through which substances may move; impermeable means that the structure does not permit a substance to pass through
Permeant – describes a substance that is able to move through a structure; impermeant means that the substance cannot pass through
semi-permeable – describes a structure through which some, but not all, substance may pass
selectively permeable – describes a living structure that is able to choose which (and when) particular substances may move through it
conductance – the ease with which a substance may pass through a structure
< EXAMPLES: A cell membrane may be permeable to oxygen but not to sodium ions, thus we say that the membrane is impermeable to sodium and that sodium is therefore an impermeant solute. Oxygen is a permeant solute. However, the cell may construct sodium channels and choose to open them under certain conditions. Thus we say that this membrane is selectively permeable to sodium. When the sodium channels open to allow sodium ions to pass through (be conducted through), we say that sodium conductance has increased. The more sodium channels open, the greater the sodium conductance. >
This membrane (yellow) allows some particles to pass through it—but not other particles.
(click image to enlarge)
Passive forms of transport
Simple diffusion
Particles pass through cell membrane as they diffuse down their concentration gradient 

Depends on how membrane-soluble the particles are
Mediated transport
Not-so-simple diffusion) 
Transporters are required
- Cellular membrane structures that serve as gateways to permit movement of particles through the membrane
- The shape & size of transporters determine what can pass through; if the shape changes, the ability to transport may change
Channel-mediated passive transport
- Particles diffuse through membrane channels
- Some channels are gated (that is, they can open and close to selectively regulate conductance)
Carrier-mediated passive transport 
- Particles diffuse through carrier mechanisms in a membrane
Also called facilitated diffusion
Osmosis
Osmosis = passive movement of water across a membrane in the presence of impermeant solutes 
- Osmosis is often described as a special type of diffusion, but is technically not diffusion even though it ACTS like diffusion
Aquaporins – water channels that facilitate osmosis
Osmotic pressure (actual vs. potential) 
- Pressure that develops (actual) or could develop (potential) when water moves (osmoses) across a membrane and changes the volumes (and thus, the pressures) on both sides of the membrane.
Isotonic – solution with same potential osmotic pressure as a cell
- There is no net water movement between an isotonic solution and the intrecellular solution inside a cell

Hypertonic – solution with higher potential osmotic pressure than a cell’s solution
- There is net movement of water from a cell’s solution INTO an extracellular hypertonic solution
Hypotonic – solution with lower potential osmotic pressure than a a cell’s solution
- There is net movement of water OUT OF a hypotonic solution and into a cell
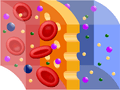
Red blood cells in solutions with different osmotic pressures.
The labels at the top of the diagram describe the solution and not the intracelluar fluid. In the figure labeled “Hypertonic” the extracellular fluid is hypertonic—but the cell’s solution has a comparatively LOW potential osmotic pressure! Likewise, in the far right figure the cell is has a HIGHER potential osmotic pressure than the hypotonic solution outside the cells. In the middle figure, the solution inside the cell has the same potential osmotic pressure as the isotonic solution outside the cell.
Notice that cells placed into a hypertonkic solution shrivel (they are said to be crenated) because water leaves them through osmosis. Cells placed in a hypotonic solution swell because water enters them through osmosis. Such cells may eventually burst (lyse).
Cells in isotonic environments experience no net movement, and thus remain normal and healthy.
The sodium-potassium pump.
Also called the Na-K pump or Na+-K+ pump, this countertransport (antiport) mechanism is found in all cells. The structure may also be called Na-K ATPase, because it breaks down ATP to get the energy for pumping ions.
The continuous opperation of this pump maintains concentration gradients across the plasma membrane for both Na+ and K+ ions. Cells tend to keep Na+ outside the cell and K+ inside the cell. The purpose of this concentration gradient will become apparent in your later studies.
Bulk transport by vesicles
Exocytosis 
- Moves large number of molecules OUT OF a cell
- Internal vesicle moves to plasma membrane and “pops open” releasing material from vesicle
- Moves large number of molecules INTO cell
- Plasma membrane pinches in, trapping extracellular material into a vesicle
- Two types:
- Phagocytosis
- Chunks are brought into cell (literally, “cell eating”)
- Pinocytosis
- Fluids are brought into cell (literally, “cell drinking”)
- Phagocytosis
- Endocytosis is often receptor-mediated
- Molecules outside the cell trigger receptors in the plasma membrane—this initiates the mechanisms of engulfing the material
- The cells “taste” before they swallow, eh?
This is a Learning Outline page.
Did you notice the EXTRA menu bar at the top of each Learning Outline page with extra helps?
Last updated: October 23, 2019 at 0:16 am
