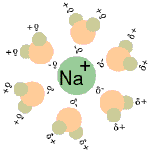Learning Outline
Introductory ChemistryPre-A&P
Chemistry is the basis for biology
Basic structural concepts
- Atom: smallest part of a pure substance, or element, that still has the properties of that substance
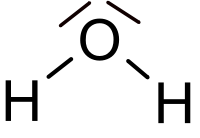
- Molecule: a particle made up of one or more atoms bound together
- Element: a pure substance, made entirely of one kind of atom (all known elements are listed in the Periodic Table)
- Compound: substance made of molecules that contain more than one kind of atom
Atomic structure and function 
- Many different models exist; none are perfect

- We’ll look at the Bohr model and electron cloud model first —then later structural and space-filling models
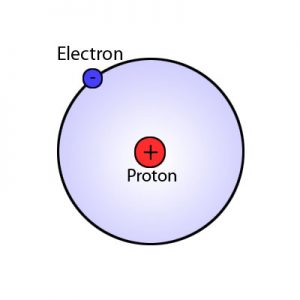
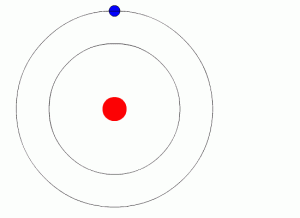
- Bohr model (named for Niels Bohr) shows electrons orbiting the nucleus like planets orbit the sun
- Electron cloud model (ECM) shows electrons as a cloud surrounding the nucleus
- Nucleus
- Protons have positive (+) charge; number of protons is “atomic number” and signifies kind of atom (which element)
- Neutrons have no charge; optional and variable in number (isotopes)
- Electrons
- Electrons have a negative (-) charge and do not affect the type of element
- Electrons may be present in regions called energy levels
- The further from the nucleus, the more energy is needed by the electron to stay there –so the higher energy levels are farther from the nucleus
- The lowest (first) energy level can contain up to two electrons
- The next few can hold up to eight electrons each (the “octet rule”)
- Energy levels fill from the inside out
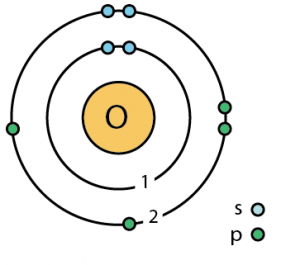
Bohr model of oxygen atom (s and p are suborbitals) Credit
Behavior of atoms: chemical bonds 

- Atoms want to be “happy”—meaning having a full outer energy level
- One path to happiness: ionic bonds
- Occurs when an atom gives one or two electrons to another atom, giving both “full” outer energy levels
- Ion: charged particle (atom or group of atoms)
- Ions “stick” together because opposite charges attract —forming a bond
- Electrolyte: molecule that dissolves in water to form ions
- Sometimes the individual, dissolved ions are also called electrolytes
- Another path to happiness: covalent bonds


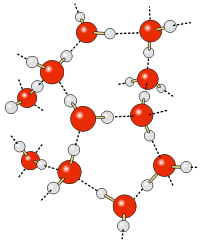
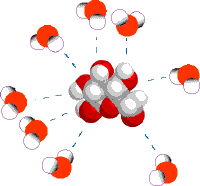
When table salt (sodium chloride crystals) is put into water, the sodium and chloride ions dissociate, and become surrounded by water molecules, forming an aqueous (watery) solution (see images to right)
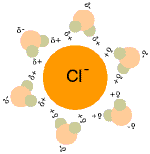
Central chloride ion (negative) forms attractions with surrounding polar water molecules, forming a solution
pH: acids, bases, and buffers 

- Water molecules may dissociate into H+ and OH–
- H+ is the hydrogen ion
- OH– is the hydroxide ion
- Solutions with equal proportion of H+ and OH– are “neutral”
- Acids: solutions with higher proportion of H+
- Bases: solutions with a lower proportion of H+ (alkaline solutions)
- Can be expressed as “power of Hydrogen” or pH
- pH scale is an inverse (base 10) logarithm of relative H+ concentration
- 7 = neutral
- acid = anything lower than 7
- base = anything higher than 7
- human blood plasma = pH 7.35-7.45
- Buffer: system of molecules that absorb or release H+ —maintaining a relatively stable pH
This is a Learning Outline page.
Did you notice the EXTRA menu at the top right of each Learning Outline page with extra helps?
Last updated: October 23, 2019 at 0:11 am